White metal looks attractive, which is especially evident when comparisons are made with yellow, gray. When making pure silver jewelry, it is impossible to achieve this effect. The color of the items will always be different from white. To improve the external qualities, a method involving the creation of a coating of another metal is used. This is . Rhodium plating silver. This method makes it possible to improve decorations by a number of parameters: increase hardness, increase resistance to mechanical damage, etc. As a result, the demand for such products increases manifold.
What is rhodium-plating silver jewelry and what is rhodium, which is coated silver
Rhodium plating is a way of improving the properties of metals that are not distinguished by their attractiveness and hardness. A thin film, a protective coating, is created on the object. In this process they use metal rhodium. It is expensive (several times more expensive than gold in costs), and therefore are not used in the production of jewelry. Their cost would be unreasonably high. For this reason rhodium is most often used to create a coating layer. It is a platinum group metal: Rh, atomic number - 45 (refers to the 9th group of elements).
Note: Rhodium is specifically used for plating jewelry because of its high reflection coefficient of electromagnetic rays representing the visible part of the spectrum (it reflects up to 80% of radiation).

Rhodium and radium: not to be confused
If we compare articles of pure Ag and jewelry that have been coated with metal by rhodium-plating, the difference will be obvious - the latter always looks more attractive. However, when studying this issue, some information about rhodium-plated silver comes into view. It should not be confused with rhodium-plated silver. These are different concepts, which imply the use of different metals.
Dangerous radium and the history of its discovery. Where is it used
Radium is a radioactive metal. It has a high production cost (due to the difficulty of obtaining it) - tons of components (coal, water, etc.) are needed to produce microscopic doses. The element of Mendeleev's table Ra was discovered over a hundred years ago by Marie Curie. The woman scientist was the first in the history of science to receive the Nobel Prize twice for her discoveries.
Maria Curie was the first to introduce the concept of "radioactivity. The term "radiation" began to be used on the wave of popularity of the poetry of Mickiewicz, who organized the "Radiation Society. Mary was his admirer. The term "radioactivity" is based on radius, which means "ray" in Latin. The woman scientist discovered that there was another element with stronger radiation than uranium. The assumption was made on the basis of a study of the properties of the tar dip from the mine. It did not contain any of the chemical elements discovered at the time. Thus began the study of the new metal.
After its discovery, radium was widely used for a variety of purposes:
- Paint for wristwatch dials;
- coating of dials of marine, aviation instruments;
- the scale of the radios;
- covering Christmas decorations, etc.
Radium was widely used because of its property to exhibit a bluish glow. Today the metal is not used as widely as it used to be. Its main use is for neutron sources. Radium is used in the production of radiological weapons. In addition, it is still used in medicine - a component of the famous radon baths. Radium is also used in defectoscopy of welds.

In the twentieth century, the popularity of radium increased. Radium water was produced - a panacea, it was drunk up to 6 times a day, facial masks were made, it was included in foods (chocolate, bread, etc.). Gradually the consequences of such use began to appear: people's teeth fell out, their jaws and faces collapsed - a consequence of the negative effect of radioactive radium, which transformed bone tissue into dust.
Applications of rhodium
The main areas of application:
- Automotive industry: catalytic effect, decomposition of combustion products into components;
- Chemical industry: production of chemical utensils, measuring equipment;
- Glass industry: production of mirrors, glass for lasers;
- electrical engineering: manufacturing of reflectors, connectors, LCD screens, etc.
The unique properties of silver. Causes of silver darkening
The main cause of black plaque is metal oxidation. This is a consequence of the interaction of the ligature in the alloy composition Ag with substances from the environment. Indirect and direct causes of color changes in jewelry:
- hormonal restructuring;
- sports;
- interaction with chemicals in detergents and cosmetics;
- high humidity.
Silver is often used in the production of jewelry. The pure metal and the alloy based on it exhibit properties:
- Reflectivity - determines the ability to reflect electromagnetic radiation falling on the surface; silver has a higher reflectivity than rhodium (95-97%);
- Softness: Ag is second only to gold in this parameter, and its softness has significantly expanded the field of application of the metal;
- malleability: silver is used to produce thin wire, and sheets of minimal thickness - it is unique propertyThe product is made in a variety of shapes and parameters;
- density: is 10.5 g/cm3which may mean that the metal is superior to iron and copper, but silver is inferior to gold and lead in this characteristic;
- discoloration: over time, Ag acquires a more pronounced yellow hue, but most often the metal reacts with other substances in an oxygen-containing environment, blackens, this is a natural process, it can be stopped by eliminating the ligature from the composition, removing all the negative factors affecting the product.

Protective metal properties and functions
When rhodium-plated silver is studied, there is an opportunity to learn more about it, for example, to understand for what purposes the protective coating is applied:
- improvement of ornamental qualities;
- Reduced consumption of gold, platinum, other precious metalsAs partly in place of them can be used silverware - more affordable, but no less attractive;
- Preventing deformation of the main product, since silver is soft;
- preservation of jewelry properties under the influence of other external factors.
The main feature of rhodium is its high hardness. It is used to create a coating of white color, and products do not lose this shade over a long period of time. Other characteristics of rhodium:
- silver-gray color;
- resistance to corrosive environments;
- loss of properties when in contact with acids, otherwise rhodium is chemically stable.
Method of application on silverware
The well-known rhodium-plated silver is produced by electroplating. This means that the precious metal (rhodium in this case) is deposited on the base material (silver) in a medium of electrolyte. This technology ensures sufficient adhesion of the coating. In addition, the durability of the jewelry increases, which is especially important because silver is soft.
If you are wondering what rhodium-plated silver is, you can make a comparison with other technologies. This method is quite different from others that also contribute to the coating. For example, you can compare it to anodized metal. This method is an anodic oxidation process.
In this case, the metal is coated with an oxide film. By comparison, oxidized items do not have an even surface. Rhodium-plated jewelry is smooth.
If you study the properties of silver, it turns out that rhodium can be used even at home. A special solution can be purchased for this purpose. It is necessary to look for it in jewelry stores. This is a solution of rhodium sulfate. The main stages of plating jewelry at home:
- Silver with rhodium is polished.
- Cleaned of metal dust, dirt, degreased.
- Dip the jewelry into the tub with the solution.
- Passing electric current.
The product is rhodiumized provided that the solution has been heated to +50°C and the voltage between the anode and cathode does not exceed 5 V.

Silver protective coating for a wide range of applications: pros and cons
When worn, silver jewelry loses its appeal. The necessity of using the precious metal to improve its external qualities is due to the fact that this metal darkens. The reason for this is the introduction of copper into the alloy. The most common cause is the interaction between the jewelry and human sweat, which contains sulfur. As a result, the items turn black.
Pros and cons of rhodium-plated silver:
Peculiarities of use and care of rhodium jewelry
Given that rhodium is not permanently applied, can cover the jewelry for a limited period of time, it is important to take good care of it.
How to care for rhodium-plated silver at home
Key recommendations:
- silverThe rhodium plating must not come into contact with cosmetics or alcohol;
- Do not wash dishes without removing jewelry;
- you can't wear rhodium-plated rings and chains during sporting activities;
- need to be flushed frequently silverware under water, if necessary, use a lint-free cloth to polish them off.
Important: Do not use ammonia or a toothbrush when caring for rhodium-plated items.

Tips for cleaning rhodium-plated jewelry with stones
Care for jewelry containing inserts (organic, synthetic), you need even more care. Tips:
- do not use abrasives, products, otherwise the stones will become turbid;
- To make rhodium-plated silver chains or rings acquire the shine that they had when they were purchased, it is usually sufficient to use a soapy solution, soak a cotton pad or rag in it, and clean the item.
Rhodium-plated silver assay
Despite the presence of a coating made with the precious metal of high value, silver belongs to the same category of products as before the application of a protective layer. It is characterized by the presence of 925 proof. The reason is the application of the thinnest layer (0.1 to 25 microns thick, which is thinner than a human hair). This could mean that rhodium-coated silver should still be sought among silverware.
How the coating affects the cost of metal
Ag is a lower-priced metal than platinum and gold. However, its prestige, appearance and properties are enhanced by the use of rhodium. In addition, this precious metal is expensive, so having a small portion of it on simple jewelry allows you to move the silver jewelry to a higher price category.
Expensive collections of famous jewelry houses
Rhodium allows us to make our jewelry "richer. But it is only in appearance; underneath the coating there is still the same Ag, so in most cases, the price of this kind of jewelry will not increase too much. Rhodium-plated silver is used even by fashion houses that make jewelry, among them: Tiffany, Christian Dior, Gucci. This material is best combined with topaz, ruby, turquoise, amethyst.

Current cost of rhodium-plated silver per 1 g
The unofficial name for a metal with a protective layer is chrome-plated silver, as it has a distinctive luster and a pure white color. You can buy quality jewelry in specialized stores of large retail chains, it is a guarantee of quality rhodium layer, which covers the jewelry. The cost of the metal:
| Price of 999 silver (Central Bank rate), rubles. | Market value, rubles. | Scrap metal price, rub. | Cost in jewelry stores, rubles. |
|---|---|---|---|
| 60 | 56 | 50 | 63 |
The graph of price changes (rubles) of 1g of rhodium-plated silver (925 hallmark) in recent months:
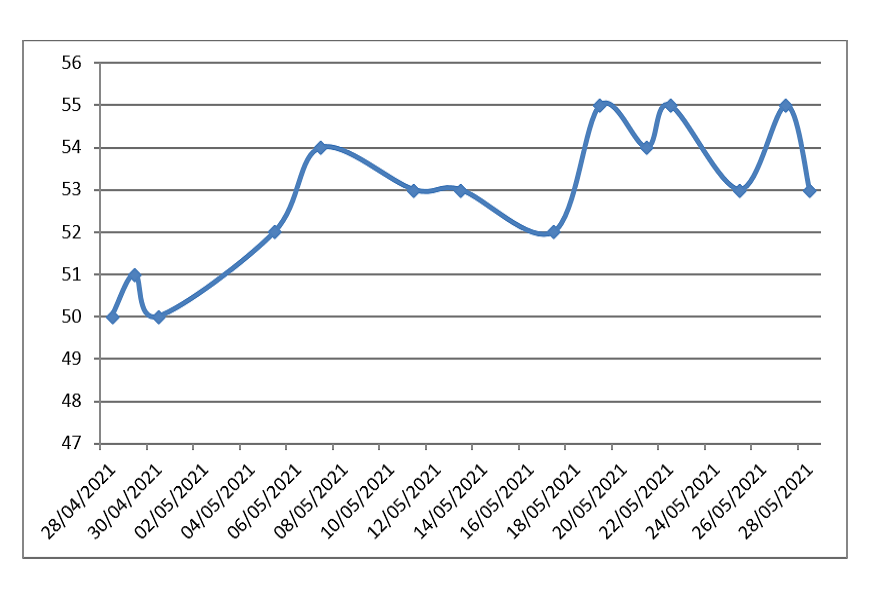
Calculation of the cost per unit of 925 proof metal
Subject: Q&A
Is there radiant silver and how is it different from rhodium-plated silver?
What is the difference between rhodium-plated silver and ordinary silver, other than durability?
How to check the authenticity of silver under a layer of rhodium?
What kind of silver is better, rhodium-plated or plain?

Oxidizing or rhodium-plating silver - which is better?
How to choose the right silver
- Examination of the branding: the jewels are stamped with a rectangular stamp, with the Ag content (92.5%) inside. For rhodium-plated items, the only option is 925;
- Request for a certificate: you may ask to see documentation proving the authenticity of the jewelry, if it contains stones, you should ask for a gemological certificate;
- Color and luster: With a layer of rhodium, the tone of the metal will be invariably white with a cool, steely sheen.
Video: What is the difference between rhodium-plated silver and sterling silver
Jeweler's comment

Radiated silver - what is it: customer reviews
I like rhodium-plated silver because of the price. It's inexpensive and looks like white gold. The shine is bright, if properly cared for, it lasts.


I wash my jewelry with a soapy solution. And it is necessary to clean silver jewelry even after jogging and application of cosmetics. Recently I read some feedback: not many people do that, but it helps to increase the life of the rhodium plating.
I don't like that the top layer of rhodium rubs off quickly. I have already had my jewelry restored twice in 3 years. The price of the service seems to be low, but if I have to do it that often, it costs a lot of money, which I am not happy with. Maybe I'm being careless with my jewelry since it's wearing off so quickly. I didn't think about it before.

