Silver - Ag or Argentum, an element of group 11 of the fifth period of the table described by D. I. Mendeleev. This simple substance is ductile metal silver-gray color. It is classified as noble, but it oxidizes in the air over time, going black. The substance has been known since ancient times and plays an important role as an industrial raw material for exquisite costume jewelry.
History of silver mining from ancient times: different meanings and uses
The conventional age of human acquaintance with silver is measured by six thousand years. This is the age of the most ancient decorationsThe metal was found during excavations in the Middle East. In Babylonian and Assyrian times, metal was considered a magical element of the moon with mystical significance. It also played this role during the Middle Ages: alchemists regarded the unusual color of the substance as the second most perfect metal and an integral part of the Great Work.
Its malleability, beauty, and relative resistance to oxidation provided another use for the metal. For the first money in the world in the form of coins was used alloy gold or silver.
For a time, the metal was valued above gold, as the latter is too malleable, and silver jewelry lasted longer.
Ratio factor prices precious metalsThe Newtonian theory, introduced by J. Newton, played a major role in the popularization of silver an important role.

In the 13th century there appeared silverware and candlesticks, later instruments, tools. Since the 19th century, when mankind mastered electricity, metalits alloys have become indispensable for the manufacture of electrical engineering.
The discovery of the chemical element, its formula and its place in the Mendeleev table. Etymology of the term and its translation
The symbol Ag is the 47th chemical element in the periodic system of Mendeleev. It is located in the 5th period. Atomic number of the element is 47, atomic weight 107.868, molar weight 107.87 g/mol.
The metal exists as two stable isotopes of atomic weight 107 and 109, as well as a radioactive isotope of weight 110.
The electronic configuration is unusual: the silver atom consists of 47 protons and 47 neutrons. The 47 electrons are arranged on 5 levels. Chemical properties depend on the structure of the last levels. This element is an exception. The calculated formula assumes 2 electrons on the 1st sublevel of the 5th level and 9 electrons on the 3rd sublevel of the 4th level. In fact, there is a slippage: an electron from level 5 shifts to level 4. This transformation causes the main valence to be 1.
In the course of its history, silver has received many names in chemistry. However, since the Middle Ages, its Latin pronunciation has been generally accepted - argentum, which translates as white, shining.
The form of existence of silver in nature. The main deposits
Mankind was so early introduced to silver because it was often found as a brilliant nugget. Even in the 15th century, large deposits were still being found: for example, a 20-ton silver block discovered in 1477 on the Schoenberg site. But today the substance is more often mined from rocks with a complex composition.
The most famous deposits are located in Mexico, Chile, China and Australia. Favorites are Peru and Poland - the share of these countries accounts for 110 thousand tons of precious metal. The U.S. also belongs to the top seven.

The reserves of the substance on the territory of Russia amount to 68 thousand tons, which makes Russia an important participant of the silver market. There are known deposits in Kazakhstan, Canada, Armenia, India, and Argentina.
The importance of European silver declined after the conquest of Peru and Argentina. Nevertheless, the extraction of the metal continued in deposits: Germany, Norway, Sweden, Austria. The pace of ore mining in Hungary, Romania and the Czech Republic did not decrease.
Natural Silver Minerals
A noble metal. It is so called not because of its beauty and luster, but because of a certain chemical inertness. It is slower to oxidize and retains its appearance longer. However, compared to gold or platinum, it seems to be active, that is why it is more often found in the Earth's crust as a complex of silver-containing rocks.
More than 50 minerals of the metal are known. Only those containing a sufficiently large proportion are used in industry. There are no more than 20 of them:
- nuggets;
- electrum - includes gold and silver;
- custelite - contains more white metal;
- argentine is a compound with sulfur;
- Prustite is a solid solution of silver, sulfur, and arsenic, quite toxic;
- bromargerite is a compound with bromine;
- Cerargyrite is a complex of chloride substances;
- pyrargyrite and stephanite - also include sulfur, antimony;
- polybasite - complemented by copper;
- Freybergite is a complex with copper, sulfur;
- argentoyarosite - includes iron;
- dyscrasite is a compound with antimony;
- Agvilarite - also contains selenium.
A side note. 70% of silver deposits refer to complex.

Physical properties of silver
The substance exhibits the typical properties of a metal. It is quite heavy, but lighter than lead. It is very ductile. It can be used to forge the thinnest wire or cloth. The crystalline lattice is face-centered, cubic, which accounts for its high electrical conductivity.
| The color of the mineral | Silver-white, turns dark gray when oxidized, then black |
|---|---|
| The color of the line | Silver White |
| Transparency | Opaque |
| Shine | Metal |
| Spiciness | No |
| Hardness (Mohs scale) | 2,5-3 |
| Durability | Moldable, malleable |
| Density | 10.1-11.1 g/cc |
| Melting point | 962 С |
| The Fracture | No |
| Radioactivity (GRapi) | |
| Magnetism | Diamagnetic |
Chemical characteristics of the element
The chemical properties are not too diverse: the substance is rather inert, as a noble metal does not dissolve with hydrochloric or sulfuric acid. But if you create certain conditions, the metal will show chemical activity.
| Reagent | Response |
|---|---|
| O2 | It does not interact with air oxygen even at high temperatures. It is possible to obtain the oxide by interaction with ozone |
| H2S + O2 | Forms a compound with sulfur, in the presence of even traces of it |
| Halogens (CL2, I2) | Oxidized to halide |
| S | The reaction proceeds by heating to sulfide |
| FeCl3 | Dissolves to form chloride |
| HNO3 | Interacts with hot concentrated acid |
The substance tends to form complex complexes with cyanides, ammonia, thiosulfates.

Studying silver as a chemical element at school in Chemistry
Silver as a chemical element begins to be studied as early as 8th grade. It exhibits typical properties noble metal, serves as a kind of model for them.
When studying inorganic chemistry, the characteristics of a substance are brought closer and the equations of various chemical reactions are parsed. Experiments with the material are revealing and interesting. However, the cost of reagents limits the number of experiments.
Wide application of the chemical properties of silver
The use of a substance is more often based on its physical properties than on its chemical properties, since its reactivity is very limited.
- For contacts of electrical products - precious metal exhibits the highest electrical and thermal conductivity. Since the 19th century, it has been used to make relay contacts, lamellae, and ceramic capacitors.
- A constituent of various solders - due to its malleability it can literally connect different materials. The metal is indispensable for soldering. Compositions with a high proportion of it are used by jewelers, with a medium proportion - in technical products, from liquid rocket engines to switches. When lead is added, silver solder replaces tin solder.
- For the manufacture of electric circuits - the substance forms solid solutions with a huge number of elements. This property is exploited in the manufacture of, for example, cathodes of galvanic elements.
- As a precious metal in the jewelry industry, it has a luxurious appeal and is in demand for the most delicate, exquisite jewelry: diadems, earrings, rings, bracelets. It is used more often in alloys with a small amount of nickel or copper: they are stronger.
- For minting coins, medals - silver money has been minted since the beginning of time. Today the material is used to make jubilee coins, to mint orders and medals.
- In photography, substance halides decompose in the light, the surface treated with them turns black. This property is used for black and white photography.
- To "disperse clouds" before a parade - spraying the skies with silver iodide causes a dramatic change in local temperature. In this way they ensure good weather at important events.
- In the manufacture of electrical engineering, electronics coated contacts and conductors in high-frequency circuits, the inner surface of waveguides. The metal provides the highest electrical conductivity.
- For mirrors, amalgam gives the mirror a much higher reflectivity than aluminum.
- As a catalyst in chemical processes - the substance accelerates chemical reactions in industrial production, for example, in obtaining epoxy from ethylene.
- The field of medicine - the antibacterial properties of silver were exploited in the times of Egypt and Ancient Greece. The metal is used to disinfect air conditioner filters, water purifiers.
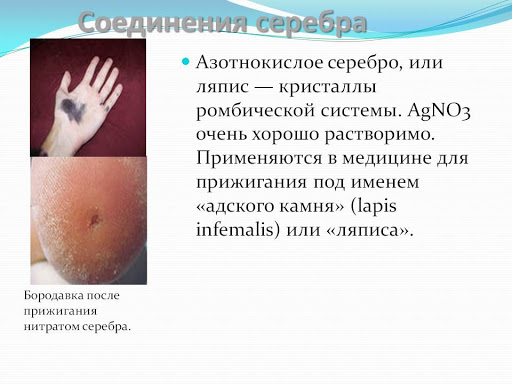
Salts resulting from the interaction of metal with acids (nitrate, chloride) are toxic and are used for medicinal purposes.

Subject: Q&A
Can I clean my water with a bar of silver?
Filters contain metal in an indelible form to prevent bacteria from multiplying inside the device.
What dose of silver is toxic?
It is quite difficult to get it. Food provides about 0.1 mg per day. This amount is easily excreted.
How do you disperse clouds with silver?
Particles of the reagent are atomized in the cloud. 1 gram of it causes billions of snowflakes to appear. As soon as a few grams of ice accumulate, the clouds turn into clouds and pour out all the water.
Where can I smelt silver at home?
Suitable are writing instruments, which in the past were often cast in alloy 800. As well as decorative objects: figurines, clock cases. A good source - solder, various old devices for transmitting messages and computer technology.

Where in nature can silver be found?
Seawater contains 0.00004 g/t of the precious substance. However, one should not attribute the antibacterial properties of seawater to the presence of Ag ions. This effect is due to the high content of salts.
Effective remedies with silver?
In medicine, it is used as a material in the manufacture of transplants. This is due to its hypoallergenicity.
Is silver used in food? Where?
Foods contain negligible amounts of Ag. Egg yolk contains the most Ag - 0.2 mg per 100 grams. You need to eat at least 30 eggs to feel the toxic effect.
Is silver a precious metal or not?
These include gold, silver, and all members of the platinum group.

How is silver marked in the Mendeleev Table?
Alchemists labeled known substances with drawings that could be identified. But compounds were named by each scientist as he or she wished. This confusion led to a reform.
Is silver a metal or a nonmetal?
- has a non-molecular structure;
- has similar properties to other metals: exact melting point, electrical conductivity, malleability, thermal conductivity;
- is characterized by a pronounced metallic luster;
- exhibits chemical reactions inherent in metals.
What metal is Ag?
The main feature is a very high electrical conductivity in the presence of oxygen. This quality has been exploited since the 19th century. Gradually, the material has been used in electrical engineering much more frequently than in jewelry.
Silver in the table of Mendeleev's name in Russian?
Is silver a heavy metal?
The density of silver is 10.5 g/cc, so the material is classified as heavy.

Is silver a pure metal or an alloy?
However, when talking about silver, we mean its alloys. Most often they contain copper. The alloys are taken to make tableware, cutlery, decorative objects and art.
A number on the Mendeleev table?
Similarly, if you know the number of an element from the table and find out which group and period it belongs to, you can determine all the properties of a simple substance with great accuracy.
Physiological effects of silver and its use in medicine
A safe amount of the substance, or rather its ions, in the blood has no physiological effect. The claim that it has disinfectant properties and healing powers is a myth, originating in Egypt and Babylon. It is a myth from Egypt and Babylon, and its safe dosage is exceeded.
In the United States and Australia, it is illegal to ascribe therapeutic properties to colloidal silver and other such products.

Food additive E174
It is ironic that a dye containing a negligible amount of Ag ions is attributed the worst properties and its ability to cause gastrointestinal disorders is pointed out. At the same time, colloidal silver, where its proportion is even higher, is ascribed healing properties.
In fact, the dye is safe because, firstly, it contains few Ag ions and, secondly, because it is rarely used due to its high cost.
Silver chemical compounds.

Russian samples of shiny metal
Silver alloys are used in jewelry and electrical engineering. This implies a certain amount of impurities. The fraction of pure Ag is denoted by a special classification, the metric system of samples.
- Less than 800 is technical silver, suitable for industrial use.
- 800 is a highly durable compound, but it tarnishes over time and oxidizes. It is called yellow silver because of its hue. It is used in the casting of crockery, cutlery, and decorations.
- 830 is a jewelry alloy that still retains a yellowish hue. It is used to make medium-priced tableware and jewelry.
- 875 - The alloy is tintless, hard and durable, but loses its luster over time. It is used for cutlery and jewelry.
- 925 -main jewelry material. The alloy is strong, does not include tints, almost does not oxidize. It is used to cast coins, medals, jewelry, and works of art.
- 960 is the plastic version. It is used to make filigree jewelry of premium quality. It is easy to damage.
- 999 is pure silver. It is used in electrical engineering. It is also used to cast bank ingots.
The grade indicates the amount of silver inside the metal. That is, 875 means that 1000 g contains 875 g of silver and 125 g of impurities.
Video: the chemical element silver, its properties and applications
Jeweler's comment

Not so long ago, the highest standard was preferred and filigree jewelry was considered the best. But in the last 5 years, costume jewelry made of the cheapest silver imitating simple ethnic jewelry has become fashionable. They are very attractive for both men and women. Colored material is combined with semi-precious stones: for example, with agate, carnelian, tourmaline.
